Around 25 years ago, Duke researchers discovered the largest known genetic risk for Alzheimer’s disease in people over 65—a gene called APOE4. Researchers still aren’t sure what it does.
These delays are not unusual for Alzheimer's research. When the disease’s namesake, Alois Alzheimer, was analyzing the brains of deceased dementia patients in the first decade of the 1900s, he noticed a bizarre jumble of plaques and tangles in their brains. The intricacies of those plaques and tangles are still being teased apart in labs today, though at a far more precise scale than Alzheimer himself could have conceived.
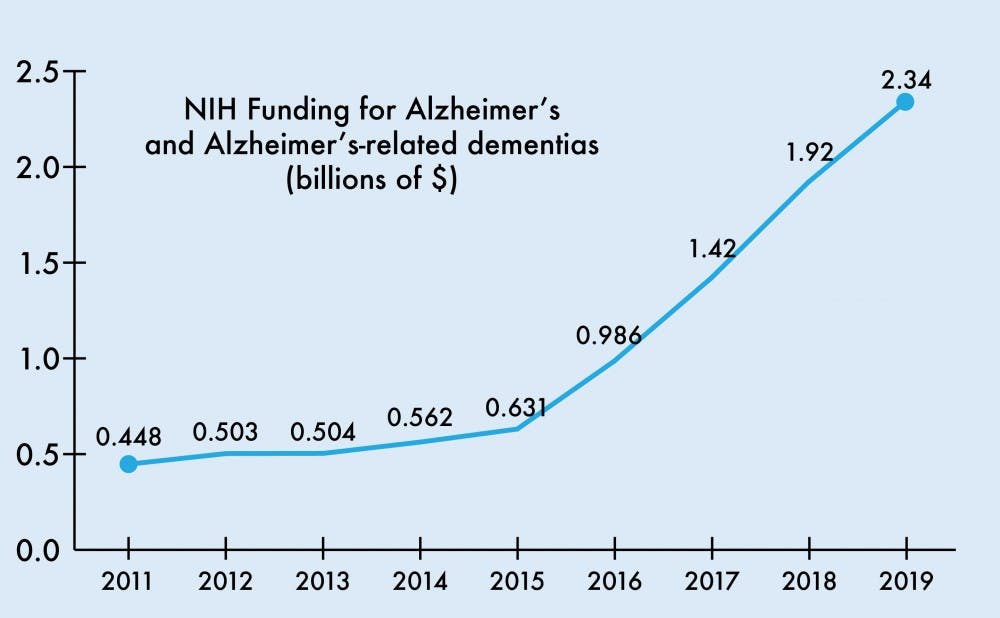
The National Institutes of Health budget for the 2019 fiscal year will raise the funding for Alzheimer’s disease and related dementia research by $425 million to $2.34 billion total. This increase continues a trend of steadily growing Alzheimer’s funding, reflecting an aging population and growing Medicare expenditures for AD.
“As the population ages, this disease will become more pronounced in terms of health burden to society,” said Ornit Chiba-Falek, associate professor of neurology at Duke. “I’m very happy that we’re getting more funding and we can investigate and hopefully advance the field.”
The Alzheimer’s Association estimates that 5.7 million Americans are living with AD in 2018, 96 percent of whom are older than 65. Women are disproportionately affected—making up approximately two-thirds of the cases—and the total number of Americans suffering from the disease is expected to climb to around 13.8 million in 2050.
‘More of the status quo’
Researchers are yet to arrive at a consensus on the molecular mechanisms driving the disease. As a result, treatments for Alzheimer’s currently focus on improving the symptoms of the disease, not directly fighting AD or attempting to reverse its course.
Richard O’Brien, chair of the department of neurology at the Duke University School of Medicine, said that the improvements brought about by these drugs are mildly encouraging at best.
“There currently are three or four drugs that are basically memory enhancers that improve your memory a tiny little bit,” he said. “They don’t do anything to the disease itself.”
O’Brien, who sees Alzheimer’s patients, cited a common memory test called the Mini-Mental State Examination. Whereas most people would receive a 30 out of 30 on the test, people with dementia would receive around 15 out of 30—and these memory drugs would bring that score to a modest 16 out of 30, he explained.
“The main thing that we do when we see them in clinic is try to problem-solve with the caregiver. What challenges are they facing? What things do we need to do to keep the faith?” O’Brien said. “That’s pretty much the state of Alzheimer’s care.”
Professor of Neurology James Burke added that these drugs may not be effective at improving memory but do yield benefits in focus and concentration.
Years have passed since the last Alzheimer’s treatment was approved by the Food and Drug Administration. The last one approved was Memantine, a drug for severe AD symptoms, which hit the markets in 2003.
Why have more than 15 years passed without another approved treatment?
“It’s not for lack of trying,” Burke said. “There’s been a large emphasis on developing treatments for Alzheimer’s disease. The problem is that all of the symptomatic and the disease-modifying therapies have been unsuccessful. There have been more than 100 clinical trials, and none of the drugs have been successfully brought to market.”
One culprit behind the lack of new treatments may be the relatively stagnant funding for Alzheimer’s and dementia research.
From 2008 to 2013, NIH funding for AD grew $92 million from $412 million to $504 million, modest compared to the upcoming $425 million increase for this upcoming year alone. The passage of the National Alzheimer’s Project Act in 2011 called for expansion of Alzheimer’s research and the first National Plan to Address Alzheimer’s Disease. The first plan set a goal to “prevent and effectively treat” AD by the year 2025.
Several years after the act’s passage, the budget allotment for Alzheimer’s and related dementias climbed to $929 million for the 2016 fiscal year and jumped to $1.42 and $1.92 billion in 2017 and 2018, respectively.
Professor of Neurology Carol Colton, who researches Alzheimer’s in her lab, praised the funding increase as an acknowledgement that more resources are necessary in the fight against AD. She credited the NIH and Alzheimer’s Association for pushing for the funding increase.
“People have decided—world governments, agencies throughout the world have decided—that we really can no longer afford both on a personal basis and a financial basis to have unabated Alzheimer’s disease,” Colton said.
Get The Chronicle straight to your inbox
Sign up for our weekly newsletter. Cancel at any time.
Burke said that the limited pool of funds researchers saw in the 2000s made it harder to compete for a piece of the pie, as grants usually got doled out to researchers with less innovative and more mainstream ideas.
“If you have something where you have a limited supply of money, it’s natural for a reviewer to think that they have to have a surefire success in order to give that money to one investigator over another,” he said. “That tends to lead to more conservative approaches—it tends to lead to more of the status quo.”
‘A thousand different theories’
O’Brien explained that the leading hypotheses for the molecular basis of the disease focus on two proteins found in abnormal states in the brains of patients suffering from Alzheimer's—amyloid-β and tau.
The amyloid protein can misfold and create plaques in the brain that are commonly associated with Alzheimer’s. Its partner in crime, tau, can become defective at its normally benign job of stabilizing cells and begin to form harmful tangles within the brain.
O’Brien added that researchers are still unsure of whether these two hallmark neurological anomalies are actually causing Alzheimer's or just byproducts of pathways gone haywire. Establishing whether amyloid and tau malfunctions are causes or symptoms remains a crucial step going forward.
“Right now, almost all the research is focused on sucking those two bad things out of the brain with antibodies, and there are a ton of trials that have gone on and are still going on,” he said.
Colton mentioned that she views the amyloid hypothesis as an “easy out” and that clinical studies have not fully supported the theory.
Despite the effort devoted to targeting amyloid, trial results have yielded little success. A 2014 study published in Alzheimer’s Research & Therapy found that clinical trials with drugs targeting amyloid had a 99.6 percent failure rate.
Colton emphasized that the amyloid hypothesis was “up in the air” and that researchers should not be focusing solely on amyloid. Instead, they should blend that angle with other ideas and theories in their investigation of AD.
“I think we need to get over all that and look beyond amyloid as the only cause of AD, because after 25 or 30 years of trying to find a way to reduce amyloid and a way to cure AD by removing amyloid, we haven’t made much progress,” she said.
Amyloid and tau, however, prove to be tempting targets for drug companies due to their relative popularity in the Alzheimer’s research field, O’Brien noted. He said that companies would rather make large investments on established ideas rather than novel ones. One such example is Biogen’s billion-dollar financing of an amyloid clinical trial.
“What pharma would tell you is the reason everything is focused on [amyloid] and tau is that’s the only two things that everyone in the field agrees on,” he said. “Beyond those two, there are a thousand different theories and no consensus around any of them.”
How Duke researchers are contributing
Researchers at Duke are studying a number of these theories in order to get closer to an eventual therapy that treats AD.
Ornit Chiba-Falek is one of these researchers. Her lab focuses on the genetic changes that can predispose people to AD in addition to how the environment can modify the genome and influence whether someone gets the disease.
In fact, Duke has been closely tied to Alzheimer’s genetics research for a number of years. It was in Duke's own Joseph and Kathleen Bryan Alzheimer's Disease Research Center where researchers pinpointed the APOE4 gene, the most significant known genetic contributor to AD in people over 65.
Kathleen Welsh-Bohmer, director of the center, explained that studying the genetics behind AD is a strength of Duke research.
“Our center has been positioned to really explore the fundamental role of genetics in Alzheimer’s disease from a variety of research perspectives,” she said.
She has recently been working on the TOMMORROW study, a clinical trial that aims to test both a genetic algorithm that may predict imminent AD risk and a new drug that could delay the onset of the disease.
The name of the trial alludes to another Alzheimer’s risk gene, TOMM40, which is located near APOE in the genome and produces a protein found in the mitochondria—cells' powerhouses. The drug being tested in the study targets the mitochondria, she explained, and results from the Phase 3 trial will be announced later in October.
Patrick Sullivan, associate professor in medicine, also studies the APOE gene in his lab. He wrote in an email to The Chronicle that approximately 25 percent of humans carries the high-risk APOE4 version of the gene.
“However, not all APOE4 carriers get AD, which means other genetic modifiers or other non-genetic risk factors (e.g. Western diet, low physical activity, chronic stress) contribute to increased risk,” he wrote.
His research focuses in part on studying the environmental reasons that determine why certain APOE4 carriers get the disease and others don’t. Another of his projects is related to developing better animal models of Alzheimer’s to test “a novel natural compound formulation” that could “get to the market much faster than conventional drugs,” Sullivan added.
Colton, another one of Duke’s AD researchers, is fascinated by the interaction between the immune system and brain. Initially, her work was met with skepticism.
At her first presentation as a postdoctoral researcher, she was prepared to speak about the presence of immune cells called microglia within the brain. However, the famous scientist who introduced the topic of the presentation—whose name she declined to give—explicitly contradicted her work by chiding that there were no immune cells in the brain, she recalled.
Scientists eventually came to accept that there were immune cells in the brain, and now Colton studies how these cells interact with the process of metabolizing food in the brain. She hypothesized that AD could be a chronic infection resulting from subtle cellular shifts in response to a virus or bacteria.
“We’ve actually come up with some very novel proteins—I’m so excited about this—that are new to AD and have not been studied in the brain,” she said. “We have recently discovered these proteins in the brain. They’re directly related to switching metabolite use from one pathway to another.”
On the road to a treatment
Eliezer Masliah, director of the division of neuroscience at the National Institute on Aging, wrote in a statement to The Chronicle that he was hopeful that the recent funding increases would advance knowledge in the field.
“We have seen remarkable increases in funding over the last several years which have helped us better understand the sequence of detrimental events and pinpoint some of the various risk factors that play a role in disease development and progression, and will help developing new biomarkers and therapies for Alzheimer’s disease and related dementias,” he wrote.
One area of increased funding is allotted for further research into genomics and personalized medicine. Another point of emphasis is the creation of “translational infrastructure programs to enable rapid sharing of data” to improve the reproducibility of research.
Several researchers The Chronicle spoke with said that expanding beyond the amyloid and tau research and investing funds in less conventional work would be the best way forward for the Alzheimer’s field.
“We oftentimes think that we know the best way money can be spent, but often, it comes out of left field,” Burke said. “I think it’s really promising to have almost another half a billion dollars to look at some of the more unusual ideas. There are lots of hypotheses about Alzheimer’s.”
He added that thanks to the additional windfall, researchers can look more into genetics, inflammatory mechanisms and metabolic pathways. Funding that focuses on amyloid and tau should continue, Burke said, but not be expanded dramatically.
Chiba-Falek added that regarding the disease as systemic—involving the entire body and not just the brain—might yield some promising clues. Additionally, it's important for scientists to follow up on previous studies, she noted.
“I think we have to come out of the box and look at Alzheimer’s disease at different levels,” she said. “We may want to see it as a more systemic disease.”
Regardless of whether some of the new theories pan out or not, Colton echoed the other researchers in explaining that funding should be devoted to areas outside the mainstream recipients of Alzheimer’s research. She added that although many of the ideas may not work, one unconventional idea that leads to an important development is worth it.
“Stopping ideas as they’re developing is not what you want to do when you have a terrible disease like Alzheimer’s disease,” Colton said.
As for whether the research will meet the 2025 goal set forth by the National Plan to Address Alzheimer’s, many researchers said that deadline will be tough to meet.
Chiba-Falek suggested that the new funding might make the target date feasible, and Welsh-Bohmer added that the progress in the last 10 years has made her “cautiously optimistic” about the goal.
However, Burke argued that the current process for drug approval makes it difficult to churn out a treatment in time.
“I think that’s an aspirational goal and not a realistic one. I think that people do better when they’re given a timeline as opposed to just an open-ended promise,” he said. “The reality of drug development is that unless you had a drug that was currently in development and pretty far along, there is no way you would have a drug out for general distribution by 2025.”
O’Brien was unsure about the 2025 time frame but predicted that AD would eventually become “somewhat preventable.”
“Once you understand what causes these diseases, those pathways will be druggable. In many people, these diseases will be prevented,” he said. “I think that prevention is probably a much more attainable target than treatment.”