Discoveries made by Duke researchers could pave the way for treatment of degenerative eye diseases.
The findings showed that cells isolated from human umbilical cords can produce molecules that help the retinal neurons in rats' eyes grow, connect and survive. Researchers, led by Cagla Eroglu, an assistant professor of cell biology and neurobiology at the Duke University Medical Center, identified one molecule in particular— thrombospondins—that are involved in the growth of connections between neurons and could allow for mechanisms to treat age-dependent macular degeneration and repair vision.
“The more we understand how these cells work, the better we can also develop similar strategies to aid patients with neurological conditions where synapses are deteriorated,” said Eroglu, who is a member of the Duke Institute for Brain Sciences.
After two years of experimentation and research, the findings of the study—which was published Nov. 25 in the Journal of Neuroscience—supported the hypothesis that the injected umbilical cells can aid the regeneration of retinal synapses in a diseased eye, therefore leading to the potential recovery of vision.
The communication between the synapses of the retina’s neurons is essential for vision.
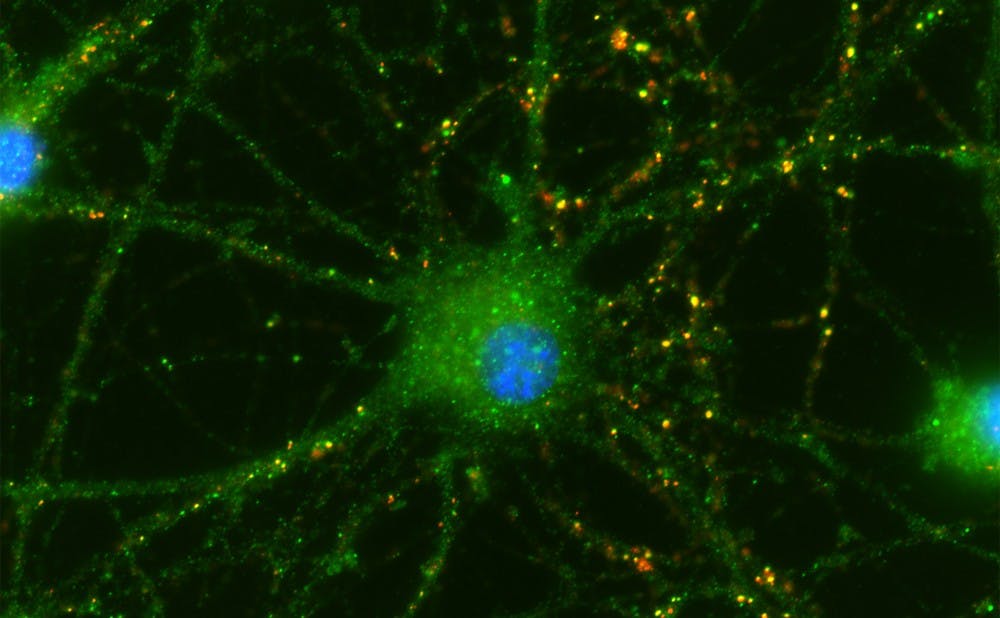
Eroglu and her associates established an experimental setup that permitted neurons and umbilical cells to bathe in the same fluid without physically touching. After a week of growing the neurons and umbilical cells in the same environment, they studied the cells to look for new connections between neurons, called synapses.
They found that retinal neurons in a bath with umbilical cord tissue-derived cells did form synapses, and sprouted tiny branches that lead to additional connections—called "neurites."
Cells also survived longer than rat neurons in a bath without the umbilical cord cells—indicating that something in the fluid was affecting the neurons.
The team identified three specific proteins from the umbilical cells—Thrombospondin (TSP) 1, 2 and 4—that are involved in the growth of connections between neurons. Deficiencies in these proteins could play a role in neurodegenerative diseases, and the lab is pursuing research in this area.
"The main purpose of the study was to unravel potential therapeutic mechanisms of these cells that will enable actual cell therapy in the future," said Sehwon Koh, a postdoctoral research associate in the Ergolu Lab and the lead author of the study. Other collaborators included Namsoo Kim, a postdoctoral research fellow in the department of psychology and neuroscience as well as Henry H. Yin, an assistant professor of psychology and neuroscience.
Get The Chronicle straight to your inbox
Signup for our weekly newsletter. Cancel at any time.