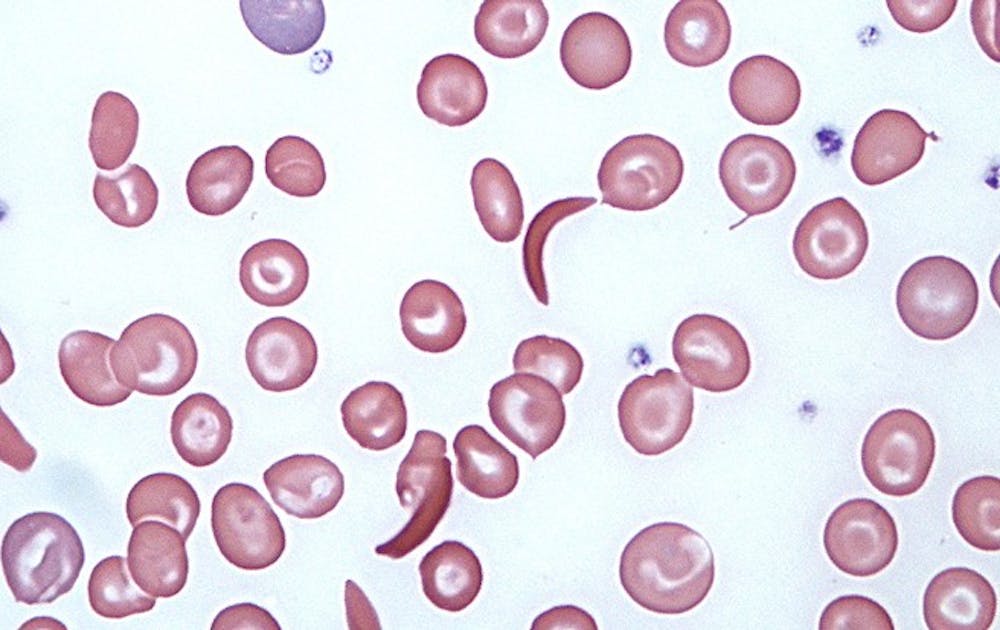
Sickle red blood cells, more commonly associated with disease, may also play a role in treating cancer tumors.
Researchers discovered that sickle cells, unlike normal red blood cells, can obstruct up to 88 percent of tumor blood vessels. When combined with chemotherapeutic agents, the sickle cells may be an effective method of attacking cancer tumors that are resistant to existing treatments. The study was published in the Jan. 9 edition of PLOS ONE and was a joint study among researchers at Duke Medicine and Jenomic Research Institute, a biotech company based in Carmel, California.
Sickle cells are more commonly known for its role in sickle cell anemia, a genetic disease that causes normal red blood cells to take on an abnormal crescent shape. In the study, researchers injected sickle red blood cells into mice with cancerous tumors. The sickle cells were found to clump in the blood vessel vessels of the tumor and its surrounding cells. In contrast, normal red blood cells moved freely through tumor vessels without sticking to one another.
“The tumor blood vessels and the sickle cells are uniquely joined at the hip,” said David Terman, head of Molecular Genetics at Jenomic. “It’s like two pieces of Velcro that are reciprocally sticky.”
The use of sickle cells is especially effective in treating hypoxic tumors, which are tumor cells that have been deprived of oxygen. These tumor cells are particularly resistant to conventional radiotherapy and chemotherapy, and this study may be the beginning of a new treatment method, said Mark Dewhirst, professor of radiation oncology at the Duke Cancer Institute.
Dewhirst added that another reason for hypoxic tumors’ resistance is because hypoxic cells do not divide. Conventional cancer treatment methods work best on cells that are dividing. Additionally, hypoxic cells are located far from major blood vessels, so drugs do not reach the tumor sites as readily.
Terman, who developed the research concept for the study in 1998, said that he brought his ideas to Duke in 2006 seeking collaboration. He currently holds the patents on the findings of the study.
“We managed to bring this project along, albeit slowly,” Terman said. “We were able to punch through the major impediments and proceed through to the endpoints.”
In order to continue research on the project, Terman noted that the next part of the research was to optimize the sickle cells so they can be more effective in attacking tumors, such as by loading the sickle cells with chemotherapeutic or tumor-killing toxins. He hopes that the study will move forward into human clinical studies within the next five to 10 years.
The study was funded by the National Institutes of Health.
Get The Chronicle straight to your inbox
Signup for our weekly newsletter. Cancel at any time.