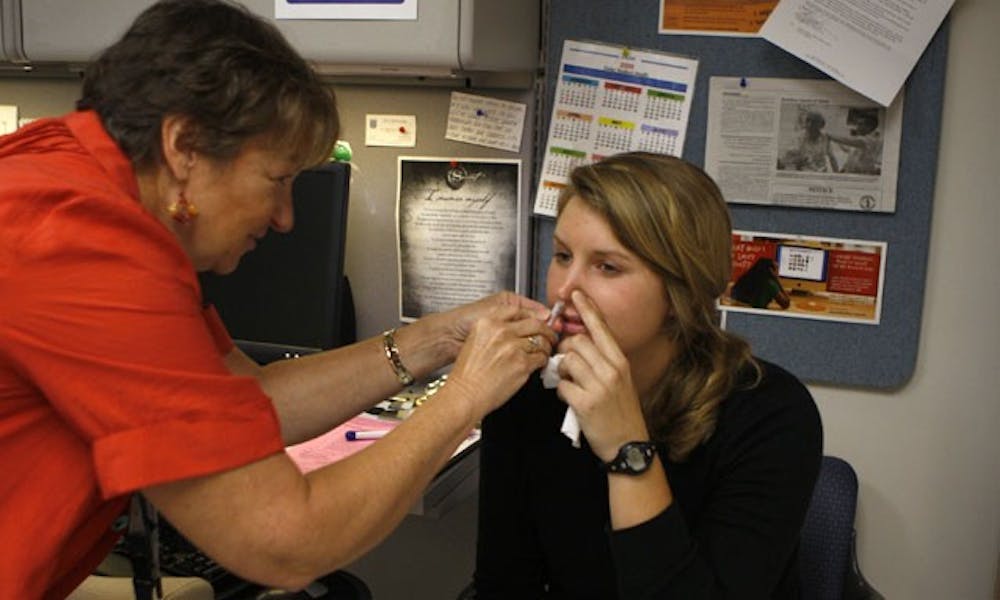
Last Wednesday, 752 students sniffed their way out of swine flu at Student Health’s flu clinic.
FluMist, a nasal spray vaccine for the H1N1 strain of influenza, arrived at the Duke University Medical Center last week. But DUMC received less than 1 percent of its requested shipment of more than 100,000 doses, a situation that was likely not different from other medical facilities, said Dr. Cameron Wolfe, an infectious disease physician at DUMC.
Currently, about 500 students have displayed swine flu symptoms, said Sue Wasiolek, dean of students and assistant vice president for student affairs. Student Health was given doses of FluMist to administer to at most 800 qualifying students who were also receiving the seasonal flu injection. The seasonal flu vaccine, which is separate from the H1N1 FluMist vaccine, was given to 1,500 students Wednesday.
“The FluMist is suitable for some patients and the injectable is suited for others,” Wolfe said.
The H1N1 FluMist is intended to treat healthy people aged 2 to 24, said Dr. Bill Purdy, executive director of Student Health.
It is a live vaccine—one that retains small components of the original virus—and is unsafe for patients with chronic health problems, weak immune systems or who are pregnant. The vaccine is also unsuitable for patients with egg allergies.
If students were not able to receive the FluMist, it was likely because they have asthma and cannot safely inhale the nasal spray, Purdy said.
“The population we’re dealing with at school is pretty healthy,” he said.
The Centers for Disease Control and Prevention recommend health care employees to be among the first to be vaccinated. But DUMC has decided not to administer the FluMist to its staff, because there is a small chance that traces of the live vaccine could infect the patients vaccinated employees are caring for. Staff members, however, will be vaccinated with the injectable H1N1 vaccine when it arrives at Duke, Wolfe said.
“We sort of sat down and we thought of our patient demographic, and... we have a high percentage of patients that are immunosuppressant,” he said.
Immunosuppressant patients, those with weak immune systems, include transplant and HIV-infected patients. Hospitals with healthier populations may have different policies, Wolfe said.
Wolfe said the decision to distribute the nasal spray first, instead of the injectable, was made at the national level, likely due to the time it takes to manufacture each form of the vaccine.
The H1N1 vaccine is federally funded and produced by four manufacturers. Wolfe said DUMC does not know when it will receive more shipments of the FluMist or when the injectable form of the H1N1 vaccine will arrive.
“I don’t hold any doubt that people who want the vaccine will be able to get it, but when they will receive it is not yet known,” Wolfe said.
Purdy said Student Health will know about a new H1N1 vaccine shipment 24 hours before it arrives.
Student Health learned they would receive the doses of FluMist the night of Oct.6, the day before the flu clinic was to offer the seasonal flu vaccine.
Jean Hanson, administrative director of Student Health, said Student Health did not want to advertise the vaccine because of the shipment’s small size—she did not want to have to turn people away. Instead, students already attending the flu clinic who met safety requirements for the vaccine were asked if they wished to receive it on a first-come, first-served basis.
“Our intent is that when they do have enough we can advertise,” Hanson said.
Although it is uncertain when more doses of the H1N1 vaccine will be available, Purdy and Wolfe advised that those who can safely receive the vaccine be treated.
“It not only reduces the chance of you getting sick, it reduces the chance of you spreading it to other people—it’s dually effective,” Wolfe said.
Get The Chronicle straight to your inbox
Signup for our weekly newsletter. Cancel at any time.